EDRN Pre/Validation Reference Set Specimen Sharing Guidelines
EDRN Standard Specimen Reference Set Guidelines (Approved March 6, 2023)
It is the goal of specimen reference sets to promote the aims of the Early Detection Research Network, i.e., testing and implementation of biomarkers useful for the early detection of solid malignancies for the purpose of improving morbidity and mortality from cancers in the population. Much of the biomarker work to date has been performed on convenience samples from cases and controls. Since these samples have been collected in a variety of ways, comparisons have been difficult. Further, cases and controls may not have been selected and matched using appropriate rigor to reduce bias. Finally, since there has not been a common resource with sufficient amounts of sample, comparison or integration of multiple markers has not been feasible. With the creation of shared and common sets of specimens from well characterized and matched cases and controls from specific disease spectra, the EDRN will overcome many of the logistic and design issues in preliminary and advanced biomarker validation. This resource will be accessible to any investigator within or outside of the EDRN after adjudication based upon a common and transparent set of criteria used to evaluate applications. It is required that results from these studies will be made publicly available.
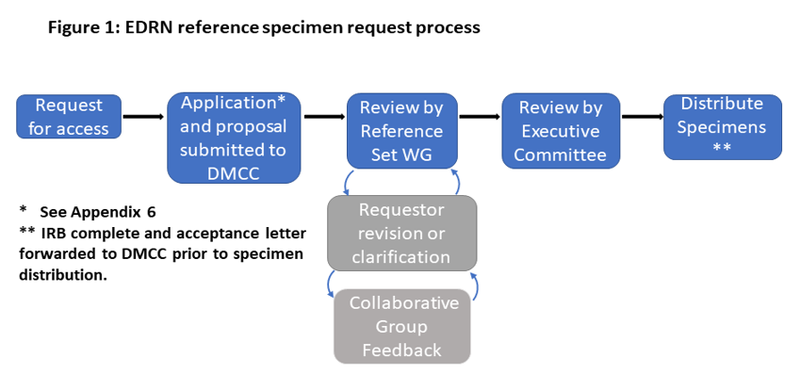
The EDRN Reference Set Working Group was created within the EDRN to draft a process and review requests through which these specimen reference sets could be accessed. It is the opinion of this committee that no completely common set of criteria could be used to evaluate biomarkers from the disparate cancers encompassed by these sets. Each cancer site has its own particular requirements, barriers, and opportunities for detection. Therefore, the appropriate organ-specific Collaborative Group will be consulted when necessary to assist the detailed scientific evaluation of applications for samples when their membership is not represented on the working group. After providing specific details related to the specimen reference set(s) being requested and institutional approval to use these sets, the requesting investigator is then expected to address the following topics as provided on the application form (see Appendix 6) in relation to their biomarker and future intentions:
- Clinical Relationship
- Background and Significance
- Preliminary Data & Methods
- Data Analysis Plan
- Collaboration
- Future Plans
If additional review criteria or application queries for any specimen reference set are stipulated by the Reference Set Working Group, the appropriate NCI Program Director will provide this additional material to the investigator. For each review conducted, it is expected that the Reference Set Working Group will include an adequate biostatistical critique by involvement of the DMCC, to ensure that appropriate consideration is given to statistical concerns of the proposal.
Upon receiving an inquiry or request regarding access to specimen reference set(s) the appropriate NCI Program Director will be notified to send an application form and any other relevant documents to the investigator. After the completed application has been returned, the Program Director will then forward it to the Reference Set Working Group. This group, in a timely manner (within one month) will review and discuss the application and offer a recommendation of whether 1) to forward the request to the respective Collaborative Group for scientific or clinical relevance review, 2) request further clarification or revision from the requestor , or 3) determine the request is of low priority or merit and defer or deny.
- If approval is given, the relevant Collaborative Group will be notified at its next monthly meeting by the Working Group chair (or co-chair). If extenuating circumstances require a timelier response, the Collaborative Group Chair will be notified by email of this decision and the Collaborative Group Chair must respond to the Working Group within 48 hours if they have any concerns. As the Executive Committee provides oversight for global EDRN activities, it needs to be informed of requests for specimen reference sets that have been received and reviewed within EDRN. In principle, however, the Executive Committee will concur with all approvals recommended by the Reference Set Working Group unless special issues are raised. NCI Program Staff will then notify the facility in Frederick to prepare the materials needed for sending the appropriate specimen reference set(s).
- If further clarification is needed, the Working Group will inform the Program Director of what concerns or questions remain with the application. The Program Director will then communicate with the investigator of these issues to ask for a resubmission.
- If deferral or denial is made, the Working Group will provide the rationale to the Program Director why the request was turned down. The Program Director will then relay this decision and its reasons to the investigator.
Appendix 5 – Policy on Blinding Specimens for EDRN Collaborative Studies
Definition of blinding:
Any information associated with the specimens remains unknown to the blinded party. Usually, the blinded party is only given the labels (coded numbers) associated with the specimens. Some assays require that cases and controls specimens are allocated with a certain ratio within the assay device. In that case, the blinded party may know the ratio of the mixing if necessary.
Unblinding may occur after the blinded party has completed the assay, completed the quality check of their data, and submitted data to DMCC, and the study group decides that the blinding is no longer necessary. Blinding may continue for other reasons (e.g., the reference set specimens that will be used for more than one study).
Studies using prospectively collected specimens:
Prospective study sites should use VSIMS and use the labels provided by DMCC. The labels provided by DMCC ensure the blinding in the subsequent uses of the specimens.
Studies using existing repository specimens:
- Principal Investigator and any personnel of in his/her lab should be blinded, regardless of whether the actual assay for the study is conducted at PI lab or at another lab. The lab performing study assays should also be blinded.
- Sites contributing Specimens should contact the DMCC for blinding guidance prior to sending out specimens to a study PI lab or assay lab and should only send out the specimens after obtaining a written permission from the DMCC.
- The study group (including DMCC) will determine whether the specimen contributing site could send the specimens directly to the assay lab without relabeling. Factors to consider include, but are not restricted to, ratio of cases and controls contributed by this the site, the nature of the study (assay done at an independent lab, or each lab will perform their candidate marker assays, etc.). If the study group decides it is necessary, relabeling will be performed prior to the shipment of specimens to assay labs.
Single Site or Collaborative Studies not Coordinated by the DMCC:
EDRN investigators are encouraged to do as much blinding as possible even at the discovery phase. For EDRN network collaborative studies, DMCC will perform blinding. For site specific studies or collaborations between sites, the blinding is done locally but investigators are encouraged to consult DMCC regarding blinding procedures.
Appendix 6 - Request for Specimen Reference Sets (on-line application)
Appendix 7 – EDRN Pre/Validation Reference Set Specimen Sharing Guidelines
It is the goal of specimen reference sets to promote the aims of the Early Detection Research Network, i.e., testing and implementation of biomarkers useful for the detection of solid malignancies for the purpose of down-staging incident cancers in the population. Much of the biomarker work to date has been performed on convenience samples from cases and controls. Since these samples have been collected in a variety of ways, comparisons have been difficult. Further, cases and controls may not have been selected and matched using appropriate rigor to reduce bias. Finally, since there has not been a common resource with sufficient amounts of sample, comparison or integration of multiple markers has not been feasible. With the creation of shared and common sets of specimens from well characterized and matched cases and controls from specific disease spectra, the EDRN will overcome many of the logistic and design issues in preliminary and advanced biomarker validation. This resource will be accessible to any investigator within or outside of the EDRN based upon a common and transparent set of criteria used to evaluate applications. It is required that results from these studies will be made publicly available.
The Reference Set Working Group was created within the EDRN to draft a process through which these specimen reference sets could be accessed. It is the opinion of this committee that no completely common set of criteria could be used to evaluate biomarkers from the disparate cancers encompassed by these sets. Each cancer site has its own particular requirements, barriers, and opportunities for detection. Therefore, the appropriate organ-specific Collaborative Group should handle the detailed scientific evaluation of applications for samples and be prepared to present the application to the Reference Set Working Group. However, it was also recognized that certain common guidelines and procedures could be developed and implemented without reducing the scientific and programmatic input of the Collaborative Groups. After providing specific details related to the specimen reference set(s) being requested and institutional approval to use these sets, the investigator is then expected to address the following topics as provided on the application form in relation to his/her biomarker and future intentions:
- Clinical Relationship
- Background and Significance
- Preliminary Data & Methods
- Data Analysis Plan
- Collaboration
- Future Plans
If additional review criteria or application queries for any specimen reference set are stipulated by the Reference Set Working Group, the appropriate NCI Program Director will provide this additional material to the investigator. The Reference set Working Group in collaboration with each Collaborative Group will determine the stringencies for granting access to specimen reference sets for which they have oversight. These standards should be established by these groups before the set(s) become available. For each review conducted, it is expected that the Reference Set Working Group will include an adequate biostatistical critique, either from within a participating laboratory of the Collaborative Group, or by involvement of the DMCC, to ensure that appropriate consideration is given to statistical concerns of the proposal.
Upon receiving an inquiry or request regarding access to specimen reference set(s) the appropriate NCI Program Director will be notified to send an application form and any other relevant documents to the investigator. After the completed application has been returned, the Program Director will then forward it to the Reference Set Working Group. The Working Group, in a timely manner (within one month) will review and discuss the application and offer a recommendation of whether 1) the investigator should be sent the requested specimen reference set(s), 2) further clarification or revision are needed, or 3) the request is deemed to be of low priority and deferred or denied.
1) If approval is given, the relevant Collaborative Group will be notified at its next monthly meeting by the Working Group chair (or co-chair). If extenuating circumstances require a timelier response, the Collaborative Group Chair will be notified by email of this decision and the Collaborative Group must respond to the Working Group chair within 48 hours if they have any concerns. As the Executive Committee provides oversight for global EDRN activities, it needs to be informed of requests for specimen reference sets that have been received and reviewed within EDRN. In principle, however, the Executive Committee will concur with all approvals recommended by Collaborative Groups unless special issues are raised. NCI Program Staff will then notify the facility in Frederick to prepare the materials needed for sending the appropriate specimen reference set(s).
2) If further clarification is needed, the Reference Set Working Group will inform the Program Director about remaining concerns or questions with the application. The Program Director will then communicate with the investigator regarding these issues to ask for a resubmission.
3) If deferral or denial is made, the Working Group will provide the rationale to the Program Director why the request was turned down. The Program Director will then relay this decision and its reasons to the investigator.
