Five-Phase Approach and Prospective specimen collection, Retrospective Blinded Evaluation Study Design
A major achievement of the EDRN was the development of a five-phase approach for biomarker development. This systematic approach to discovery, development and validation is used to help identify “winners” or “losers” among biomarkers.
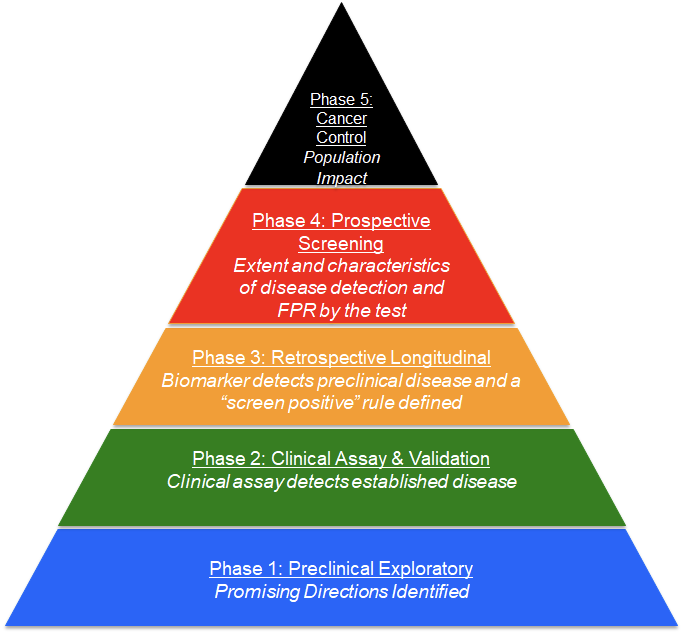
The phased process has been widely accepted by the biomarker research community. The multi-step approach includes: Phase 1 – Discovery; Phase 2 – Clinical Assay and Validation; Phase 3 – Retrospective Longitudinal; Phase 4 – Prospective Screening; and Phase 5 – Cancer Control.
This rigorous, efficient method for biomarker discovery and validation, which is used throughout the EDRN, requires a highly collaborative organization that includes a broad range of expertise with each collaborator having an essential role. The organization has been compared by some to the mission control room of NASA, where if a single component in a pre-launch sequence is not performing at 100%, the launch is “held” until the issue is resolved. Similarly, because of the essential nature of each of the components in the process of biomarker validation, all components are regarded as equals.
The broad range of individuals involved, from biostatisticians to clinicians, scientists, and technology experts, are equally valued and respected within the organization. In this context, it has been the entire team that ensures and celebrates each success, rather than an individual.
EDRN has also developed and adopted the Prospective specimen collection, Retrospective Blinded Evaluation study design, an approach to reduce bias during all phases of biomarker discovery and validation. The Prospective specimen collection, Retrospective Blinded Evaluation study design has four key elements: clinical context and outcomes; criteria for measuring biomarker performance; the test itself; and the size of the study.
Critical features of this design are: all patients are enrolled prior to diagnosis; cases and controls are enrolled under the same conditions; and all samples are collected and processed identically. For a screening application, this design may require large numbers of study participants because the incidence of most cancers is low. For instance, as the incidence of colon cancer in a screening population is about 0.5 percent, 20,000 patients would need to be enrolled to obtain 100 cancers.
