Mission and Structure
The mission of the Early Detection Research Network (EDRN) is to discover, develop and validate biomarkers and imaging methods to detect early stage cancers and to assess risk for developing cancer, and to translate these biomarkers and imaging methods into clinical tests. A consortium of more than 300 investigators at academic institutions and in the private sector are working collaboratively to bring biomarkers and imaging methods to clinical use.
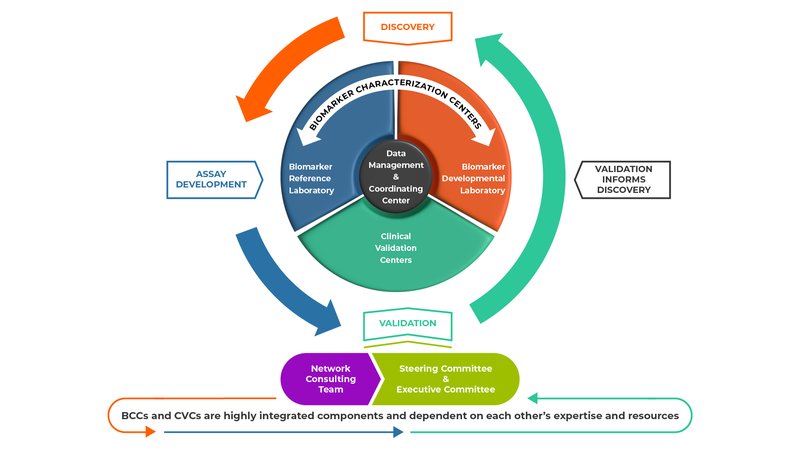
The major components of the EDRN (Biomarker Characterization Centers, Biomarker Developmental Laboratories, Biomarker Reference Laboratories, Clinical Validation Centers, and Data Management and Coordinating Center) have distinct but complementary roles and work synergistically to facilitate the discovery, development, and validation of cancer biomarkers.
Biomarker Characterization Centers (BCCs): BCCs characterize new biomarkers or refine existing biomarkers. There are two kinds of BCCs:
- Biomarker Developmental Laboratories (BDLs): BDLs discover and develop new biomarkers or refine existing biomarkers. They are the primary source of new biomarkers or panels of biomarkers on which the EDRN conducts validation trials. They also develop assays to detect candidate biomarkers and conduct pre-validation studies.
- Biomarker Reference Laboratories (BRLs): The primary role of the BRLs is to conduct assays for EDRN validation trials. The assays are performed on blinded biospecimens to minimize bias in the analysis and independently verify the assay performance. BRLs also serve as the primary resource for analytical validation of biomarkers, technological development, standardization, assay refinement and quality control.
Clinical Validation Centers (CVCs): The primary role of the CVCs is to conduct validation trials on biomarkers discovered/developed by both EDRN and non-EDRN investigators. CVCs also provide high-quality, well-annotated biospecimens to the BDLs for biomarker discovery, development and pre-validation studies. The use of biospecimens collected using rigorous standard operating procedures helps minimize false discoveries.
Data Management and Coordinating Center (DMCC): One of the major roles of the DMCC is to work with the CVCs to conduct biomarker validation trials. The DMCC assists with protocol design, monitors the trial, and maintains the data and biospecimen tracking system. The DMCC is responsible for analyzing the results of the trials, thereby reducing bias as they are independent from the laboratories that discovered the biomarkers. The DMCC provides statistical advice to the BDLs, develops theoretical and applied approaches for simultaneous analysis of multiple markers, and collaborates with the EDRN Informatics Center.
EDRN Steering Committee: The EDRN Steering Committee is composed of all the EDRN Principal Investigators (PIs) and is responsible for overseeing the activities of the EDRN and setting priorities. The Steering Committee meets in person twice a year. The Executive Committee, which consists of the EDRN Chair and Co-Chair and the elected Chairs of the Collaborative Groups, have monthly conference calls.
In addition to the formal structures, the network encourages cross-collaboration through several channels:
Collaborative Groups: Within the EDRN there are four organ-specific Collaborative Groups—Breast and Gynecologic Cancers, Colorectal and Other Gastrointestinal Cancers, Lung and Upper Aerodigestive Cancers, and Prostate and Other Urologic Cancers.
All EDRN PIs, co-investigators and many Associate Members are members of one or more of these collaborative groups. They have monthly conference calls and meet twice a year in person to update each other on their progress and to develop collaborative projects that use the resources of all the investigators. These projects frequently involve comparing the performance of biomarkers from different laboratories in a common set of biospecimens and when appropriate combining these biomarkers to create a panel. These projects are supported by the grantees' set-aside funds, which are restricted for this purpose.
Interagency Agreements: The EDRN has collaborations with four other federal agencies: the National Aeronautics and Space Administration's Jet Propulsion Laboratory (JPL), which supports EDRN informatics and data science; Pacific Northwest National Laboratory (PNNL), which supports the development of proteomic-based assays; the Department of Defense Center for Prostate Disease Research (CPDR), which provides valuable biospecimens collected from subjects with prostatic diseases with high representation of African Americans; and the National Institute of Standards and Technology (NIST), which assists in the development of standards and references.
